This winter, Pam and David Catcheside continued their surveys of the fungi on Kangaroo Island after the catastrophic fires of 2019 and 2020. Reports on previous findings were published in these blogs:
- Fire blog 4: Fungi and fire
- Preliminary studies of the fungi in Flinders Chase National Parks after the 2020 fires
After a brief, preliminary survey in April, we considered that July would be optimum for the main survey, which would include Dr Teresa Lebel, Senior Botanist at the State Herbarium of South Australia, Helen Vonow, Curations Manager, and Julia Haska, an Herbarium Research Associate. However, a COVID lockdown aborted that trip: Julia was already on the island, but just as Teresa and Helen arrived at Cape Jervis, well ahead of time for the noon ferry, they learned a lockdown would start at 6pm. Pam and David were en route at Lucky Bay so as a small compensation we all met up at Deep Creek Conservation Park to look at fungi there, before heading home in time for the curfew. The variety and abundance of fungi at Deep Creek made it even more galling that we were not able to explore the fungal offerings of Kangaroo Island, though we did make some interesting collections.
Although Teresa, Helen and Julia were unable to join us, we were able to schedule a week-long KI trip in late August. However, as the fungal fruiting season in South Australia is usually from June to early August, we knew we had missed the optimum time. An additional constraint was that some sites we usually survey were off limits due to feral pig control operations.
We recorded 35 species of fungi, making collections of 27 of these. One third (10 species) were fire-associated, pyrophilous species and included disc, stonemaker, as well as wood-rotting fungi.
Disc fungi
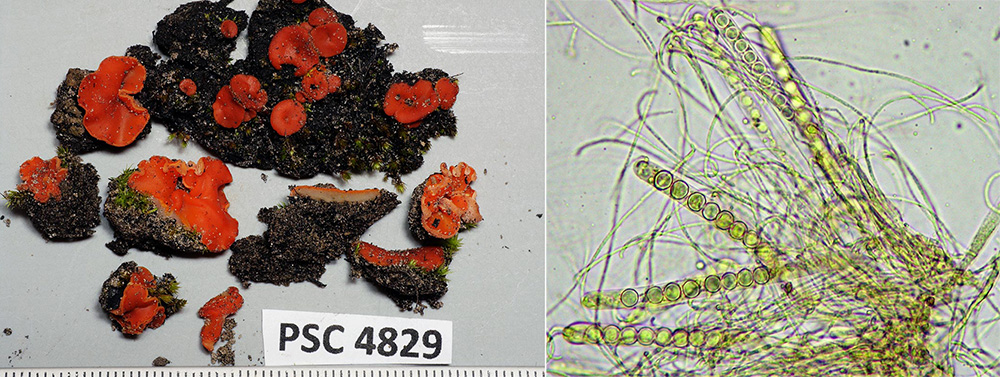
Disc fungi are amongst the early colonisers, forming mats over the soil surface, helping to bind soil particles and reduce erosion. We found a few patches of the orange Pyronema omphalodes (above) and scattered groups of Pulvinula, whose bright orange discs with their paler, smooth and hairless undersides stand out against the burnt soil.
Pam never trusts the outward appearance of the majority of disc fungi, recognising that the microscopic characters of ascospore size and number, ascus size and shape and features of other cell structures are necessary to determine species. Indeed, each of the four collections of Pulvinula that were made differed in some of these characters, suggesting they belong to different species, two of them possibly undescribed. Planned molecular work will clarify this.
Other pyrophilous disc fungi such as species of orange Anthracobia and black-brown Plicaria and Peziza, found after the 2007 fires and in 2021, were absent. It is possible that these had fruited early this year in June or July but it appears probable there is a lower abundance and fewer species of pyrophilous discs than after the 2007 fires.
Mycorrhizal fungi
Mycorrhizal fungi are essential in all forest ecosystems, providing minerals and water to their partner tree. Laccaria species, common colonisers of land cleared by fire or other disturbance, were present, but few other mycorrhizal fungi were found — just a few specimens of Amanita and Cortinarius and an earthball, Scleroderma albidum (right).
Mycorrhizal fungi are expected to be affected when their symbiotic plant partners are killed by fire (Dove & Hart 2017) so it is not surprising that they will be depleted following fire. Again, it is possible that they fruited earlier but it is of concern that we found so few of these important fungi.
Wood-rotting fungi
Fungi are responsible for much of the breakdown and recycling of organic matter, releasing nutrients and making them available for new plant growth (Ingold & Hudson 1993; Crowther et al. 2012). After the summer fires of 2019-2020 there was plentiful substrate: large quantities of fallen bark, dead branches, logs and trunks were at all sites. Rainfall on Kangaroo Island in 2021 had been high and we had expected to find fluffy mycelium and patches of flat ‘paint’ fungi on some of the wood. However, in spite of our careful examination, even the lowest and dampest layers of the piles of fallen bark showed little evidence of the usual ‘rotters’. We surmised that the fungal inoculum was insufficient to start breakdown of the bark.
]We did find a few wood-rotting bracket fungi. One of the most common was Porodisculus pendulus, which grew in extensive swarms up the burnt trunks of eucalypts. This is a small hoof-shaped pored bracket and the specimens were much tinier, less than half the size, than those we had found after the 2007 fires. We also noted the lilac bracket, Rhodofomitopsis lilacinogilva, and shelf fungus, Stereum hirsutum, but these are not considered to be obligate fire-associated wood rotters.

Wood rotting fungi: Porodisculus pendulus on burnt eucalypt trunks (top), Rhodofomitopsis lilacinogilva (middle), and Stereum hirsutum (bottom). Photos: David Catcheside.
Dung fungi
On a brighter note! One little fungus that was in abundance was Poronia erici. It was growing mostly on kangaroo dung but it also grows on the dung of other marsupials. It forms hard white button-like discs punctuated with tiny black pores which communicate with minute ascus-containing flasks embedded in the disc. Spores are released through the narrow necks of these flasks. The presence of this fungus is a positive sign that marsupials are returning to the burnt sites on Kangaroo Island and possibly reintroducing fungi lost to the fire.
Monitoring of fungal succession after fire and re-establishment of pre-fire fungal assemblages may inform better management of the environment after fire. It was unfortunate that COVID-19 prevented us from a proper assessment this year.
Further reading
- Atlas of Living Australia. Pulvinula Boud.
- Catcheside, P. (2009). Phoenicoid discomycetes in Kangaroo Island. Fungimap Newsletter 38: 5-8.
- Crowther, T., Boddy, L. & Hefin Jones, T. (2012). Functional and ecological consequences of saprotrophic fungus–grazer interactions. ISME Journal 6: 1992–2001.
- Dove, N.C. & Hart, S.C. (2017). Fire reduces fungal species richness and in situ mycorrhizal colonization: a metaanalysis. Fire Ecology 13(2):37–65.
- George, P. (2008). Fungimap survey on Kangaroo Island. Fungimap Newsletter 36: 13-15.
- Grgurinovic, C.A. (1997). Larger Fungi of South Australia. (The Botanic Gardens of Adelaide and State Herbarium and The Flora and Fauna of South Australia Handbooks Committee: Adelaide).
- Ingold, C.T. & Hudson, H.J. (1993). Ecology of saprotrophic fungi. In: The Biology of Fungi, pp. 145-157. (Springer: Dordrecht).
- McMullan-Fisher, S.J.M., May, T., Robinson, R., Bell, T., Lebel, T., Catcheside, P. & York, A. (2011). Fungi and fire in Australian ecosystems: a review of current knowledge, management implications and future directions. Australian Journal of Botany 59: 70-90.
Contributed by Pam Catcheside (State Herbarium Hon. Associate)
& David Catcheside (Flinders University).





















You must be logged in to post a comment.