In 2016 and 2017, State Herbarium of South Australia botanists participated in two Bush Blitz expeditions to Lake Torrens and the Great Victoria Desert. The two reports on the collections and findings about plants, fungi and algae were submitted to Bush Blitz soon after the field trips. Part of the information was presented in the official Bush Blitz survey reports, but not the full data.
The detailed reports on both expeditions are now available:
(1) Lang, P.J., Kellermann, J., Bell, G.H., Brodie, C.J., Vonow, H.P. & Waycott, M. (2018). Lake Torrens Bush Blitz survey: Vascular plants, cyanobacteria, algae, bryophytes, lichens and macrofungi. Report for Bush Blitz, Australian Biological Resources Study, Canberra. (State Herbarium of South Australia: Adelaide). (5.1mb PDF).
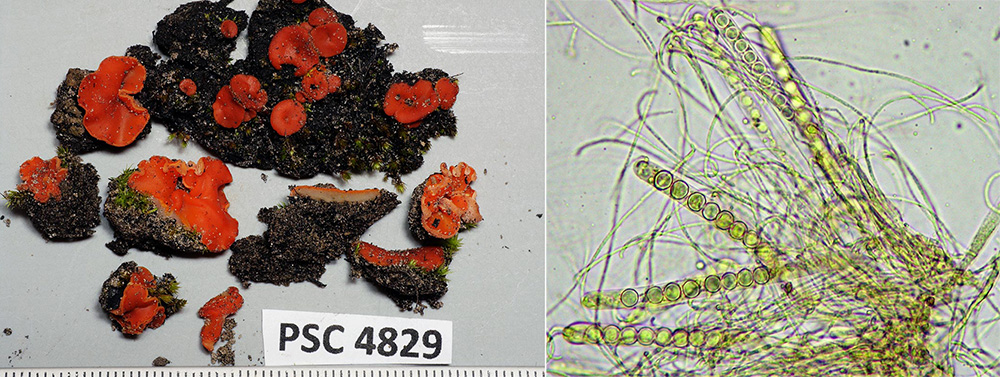
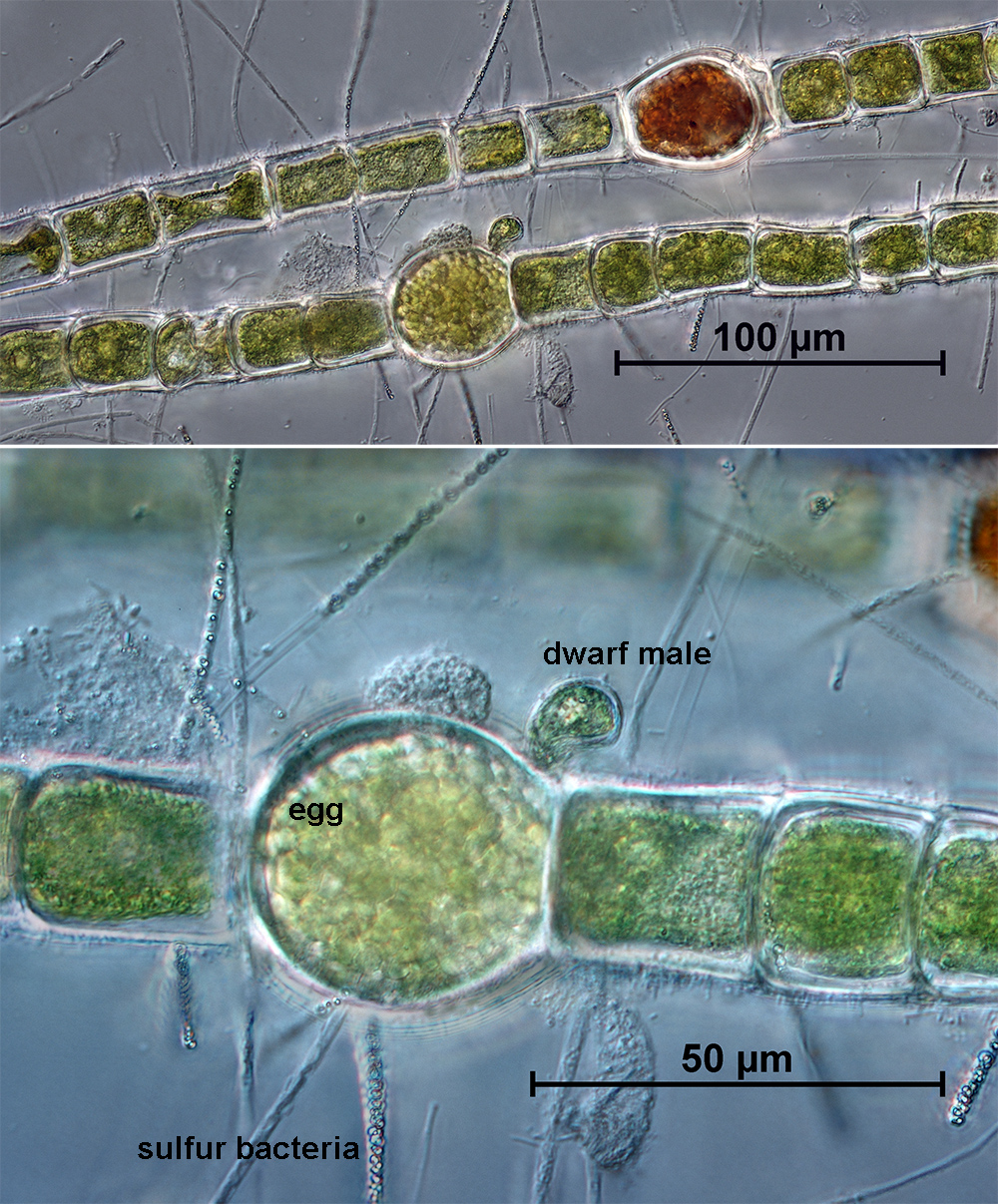
The 2016 Bush Blitz Survey to Lake Torrens and five adjoining pastoral leases provided an opportunity to greatly increase the knowledge on the flora of the area. The preceding seasonal rainfall provided high quality conditions for plant growth and flowering and also the presence of water in areas of Lake Torrens and surrounds. A total of 382 unique taxa were recorded on the survey, comprising 358 vascular plants, 1 bryophyte, 4 algae, and 7 cyanobacteria; 12 lichens were also recorded.
Significantly, 32 vascular plant taxa and 6 cryptogams (1 bryophyte, 3 cyanobacteria, 2 algae) were recorded from the study area for the first time.
Including previous records, this resulted in a total of 699 vascular plants for the survey area, of which 30 are introduced, weedy plants. Five of the weed taxa are highlighted as needing control measures while they are still in low numbers. The remaining 25 weed taxa are of low concern, but should be monitored. In total, 60 cryptogams are recorded for the area, including 18 bryophytes, 4 algae, 7 cyanobacteria, 12 lichens and 19 fungi. Non-lichenous fungi are covered in a separate report by T. Lebel, but the 16 taxa recorded on survey are also listed in Appendix III.
The survey resulted in the collection of 996 specimens and complementary observational records. Leaf samples in silica gel for future DNA analysis were collected from almost all specimens.

The Botany Team processing plants at McCormack Reserve, Roxby Down Station, during the Lake Torrens expedition. Photo: P.J. Lang.
(2) Lang, P.J., Kellermann, J., Bell, G.H., Canty, P.D. & Waycott, M. (2019). Great Victoria Desert Bush Blitz: Vascular plants, bryophytes, lichens and macrofungi. Report for Bush Blitz, Australian Biological Resources Study, Canberra. (State Herbarium of South Australia: Adelaide). (2.4mb PDF).
The 2017 Bush Blitz Survey to the Great Victoria Desert (GVD) targeted Mamungari Conservation Park (CP) and the adjoining area of the Maralinga Tjarutja Lands. It provided an opportunity to greatly increase the knowledge on the flora of the area.
The survey resulted in the collection of 660 specimens with nearly all the vascular plant collections having duplicate samples for PERTH herbarium plus leaf tissue samples in silica gel for future DNA analysis. The collections comprise 539 vascular plants, 25 bryophytes, 18 macrofungi and 78 lichens. These represent 358 unique taxa (excluding hybrids and intergrades), comprising 319 vascular plants, 9 bryophytes, 12 macrofungi and 18 lichens.
A validated checklist for the area was compiled, incorporating ALA specimen based records. The total number of accepted vascular plant taxa is 529 for the GVD study area and 436 for Mamungari CP. The Bush Blitz Survey resulted in a total of 48 vascular plant taxa beingcollected from the study area for the first time, with two of these also new records for South Australia (SA).
The checklist for cryptogams comprises 73 taxa (20 bryophytes, 19 fungi and 34 lichens), but due to limitations of available historical data this is not a definitive list for those groups. Four of the cryptogam taxa collected on survey are potentially new to SA.

Escarpment at the western edge of Serpentine Lakes in Mamungari Conservation Park, Great Victoria Desert. Photo: P.D. Canty.
The official Bush Blitz Survey Reports of the two expeditions with lists of recorded plants and animals, as well as an overview map, are available here:
-
- Great Victoria Desert (2017)
- Lake Torrens (2016)
Reports for previous Bush Blitz expeditions in South Australia can be accessed here:
-
- Hiltaba Nature Reserve and Gawler Ranges (2012)
- Bon Bon Station Reserve (2010)
- Witchelina Reserve (2010)
Compiled by State Herbarium botanist Jürgen Kellermann.

 Since 2017,
Since 2017, 








You must be logged in to post a comment.